Tyler Ford
04/28/2021
As discussed in a previous blog post, there are many different ways to get CRISPR systems into cells. Once there, they can carry out a variety of functions including genome editing, gene regulation, and much more. Effectively getting CRISPR systems into cells, however, is key.
In this blog post, we’ll describe some of the important factors scientists consider when choosing a CRISPR delivery system. Having access to a diverse set of CRISPR tools gives scientists the versatility to address some of these factors and choose among many possible CRISPR delivery techniques. In our next blog post, we’ll cover some of the more popular CRISPR delivery methods. For more thorough reviews of CRISPR delivery see Wilson and Gilbert 2018, Yip 2020, Haasteren 2020, and Bulcha et al 2021.
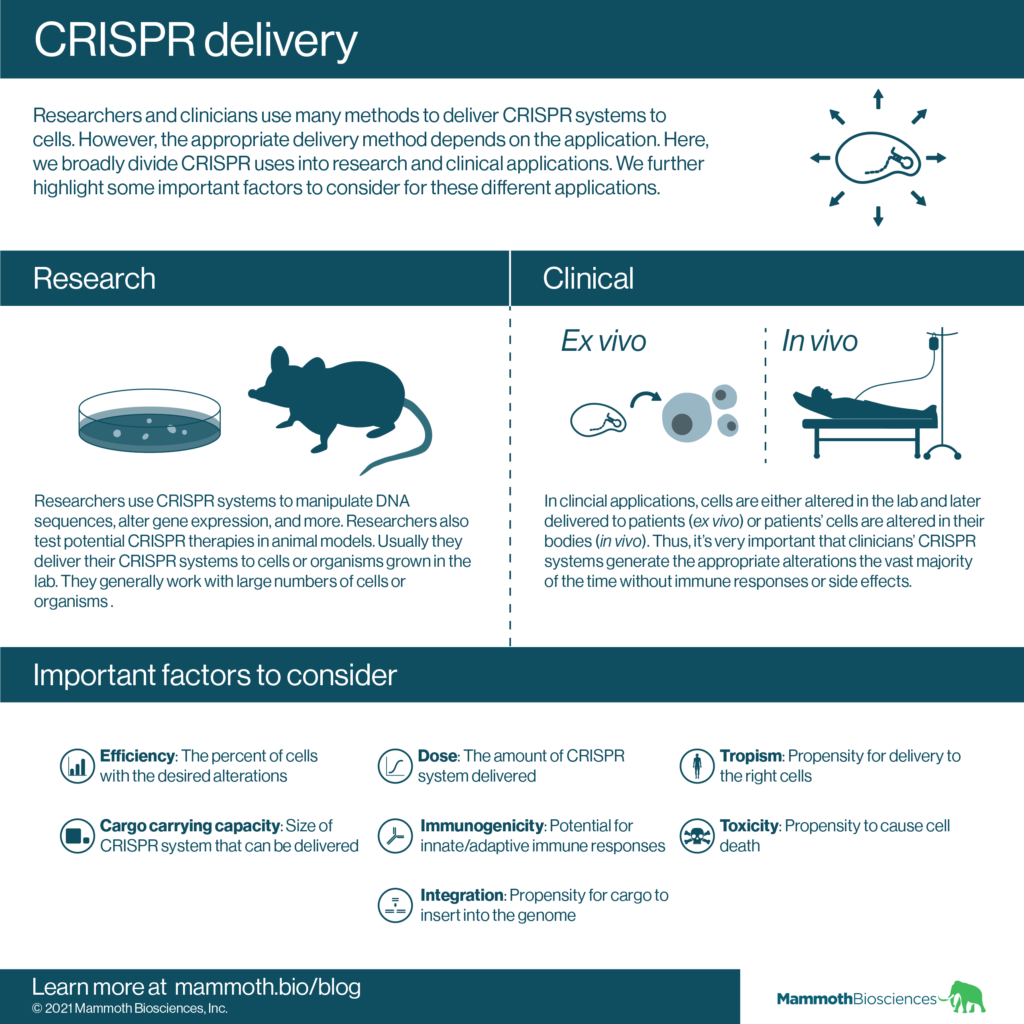
Research delivery needs vs clinical delivery needs
Scientists’ CRISPR delivery needs differ depending upon whether they’re doing research or clinical work.
In research, scientists are often willing to spend the time required to find the cells where delivery was successful. For example, if performing a genome editing experiment on a pool of cells in a dish (in vitro), scientists may spend time specifically looking for the small number of cells that have the appropriate edits. They can then use these cells for a wide range of other experiments.
There are many CRISPR clinical trials underway and things work a little bit differently in the clinic. Generally, clinicians would like their delivery methods to be as efficient and safe as possible. And, even in the clinic, we can divide CRISPR therapies into two broad groups that have different delivery needs:
- Ex vivo therapies- Here CRISPR systems alter cells outside the body. Clinicians later administer the altered cells to patients. As such, in these therapies, scientists deliver their CRISPR systems to cells grown or maintained in the lab. This is useful because scientists can specify certain conditions in the lab that might make delivery easier. They can also selectively grow and maintain exactly the cells that they want to alter. The cells’ alterations may do such things as correct a genetic abnormality or make the cells particularly good at fighting cancer.
- In vivo therapies- Here scientists use CRISPR systems to alter cells in patients’ bodies. Thus, they must deliver their CRISPR systems directly to the patient. In these cases, it may be more difficult to deliver CRISPR systems to the right cells in the right body part. Clinicians also have to deal with problems such as immune responses to the delivery method and to the CRISPR system itself.
While in vivo therapies face many challenges, some diseases can only be treated with in vivo therapies. For example, clinicians may need to deliver their CRISPR systems directly to cancer cells in a tumor or to defective cells in the liver to treat their associated diseases.
Important factors to consider in CRISPR delivery
We will cover some of the popular chemical/physical and viral delivery methods in the next post in this series. For now, we’ll provide a broad overview of some of the factors researchers and clinicians consider when selecting a delivery method. Please keep in mind that this is not an exhaustive list. The most important factors depend on scientists’ needs and the needs of their experiments.
Some important factors to consider when choosing CRISPR delivery methods include:
- Efficiency – By efficiency we mean how often cells are effectively targeted by a CRISPR system. For instance, a measure of efficiency could be the fraction of cells in a dish that are accurately edited by a CRISPR system.
Efficiencies can vary widely across cell types, organisms, and specific delivery techniques. Therefore, we won’t make broad generalizations here except to say that in most research applications high efficiency is not as important as it is in the clinic. Researchers can generally find even a small fraction of cells that have been effectively targeted and enrich them for later experiments. Therapies in the clinic require effective targeting of as many cells as possible. - Dose – By dose we mean how much of a CRISPR system scientists delivery and how long it remains active. If too much of a CRISPR system is active for too long, off-target alterations may start popping up. Proper dosing is generally more important in the clinic.
- Tropism – This is the propensity for a delivery method to deliver its cargo to particular cell types. Tropism is less of an issue in in vitro and ex vivo work than in vivo work; scientists can specifically isolate the cells they wish to alter for in vitro and ex vivo work. Nonetheless, even in in vitro and ex vivo work, some cell types may be more amenable to certain types of delivery than others.
- Cargo carrying capacity – This is the ability of a particular delivery method to carry CRISPR systems of different sizes. Viruses have well-known carrying capacities. Lentiviruses can carry the largest cargoes, then adenoviruses, and then adeno-associated viruses (AAVs).
- Immunogenicity – By immunogenicity we mean the propensity of a delivery method to trigger adaptive or innate immune responses. Activation of the immune system can cause rejection of the delivered cargo. This may lead to ineffective cellular alterations and/or dangerous side effects. Immunogenicity is generally not an issue when working with cells in a dish, but it may be an issue when working with full organisms. Immunogenicity is a very important factor to consider in in vivo therapies.
- Toxicity – Some delivery methods use chemicals or treat cells in ways that are toxic. These can result in cell death. Lower toxicity is generally better across all settings.
- Integration – Many methods deliver CRISPR systems encoded in DNA. DNA always has the potential to insert (integrate) into the host cell genome. This can result in prolonged production of the CRISPR system. This may lead to off-target alterations and/or may elicit immune responses. Integration itself may lead to unintended changes to target cells (e.g. integration can cause cells to become cancerous as highlighted in Hacein-Bey-Abina et al 2008). Thus integration is generally more of a concern in the clinic than in research.
Again, all of these factors should be considered for all CRISPR experiments. In addition, researchers and clinicians may have to consider many other factors. Overall, we hope this post gives you a good overview of the things researchers think about when choosing a CRISPR delivery method.
The Cas protein – delivery connection
One of the reasons we’re developing novel Cas proteins is so scientists at Mammoth and elsewhere have the versatility to pair the appropriate delivery methods with the appropriate Cas proteins. In doing so, they may alleviate some of the concerns associated with the factors above. For instance:
- Cargo capacity too low – scientists can use smaller Cas proteins like Cas14 and CasPhi
- Cas9 immunity issues – scientists can work with Cas proteins that are less likely to elicit immune responses
- Cas9 specificity issues – scientists can work with Cas proteins with higher specificity
With many different Cas proteins and delivery methods, hopefully researchers can find combinations that suit their needs!