Tyler Ford
10/04/2019
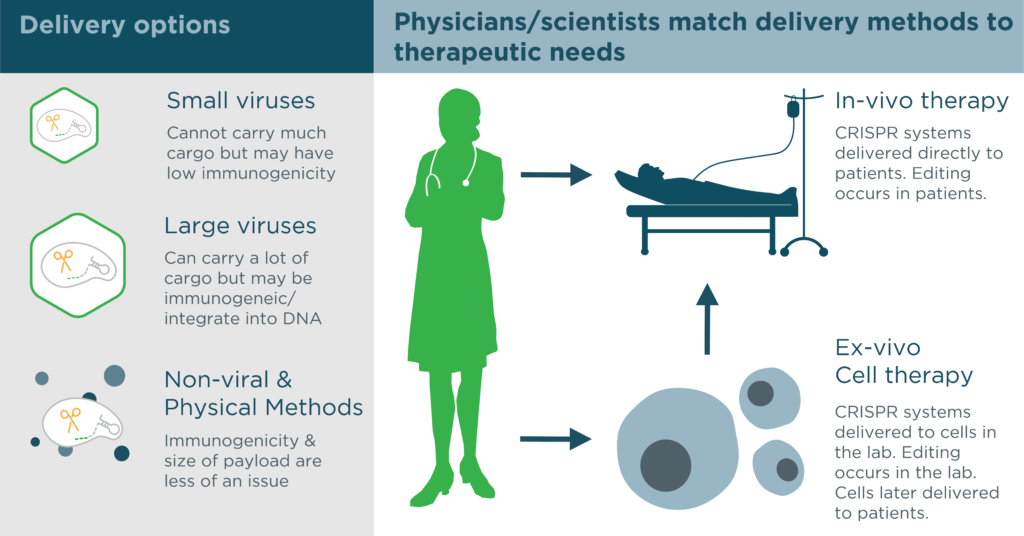
Cell and in-vivo gene therapies are at pivotal points in their development. Both types of therapy alter DNA to treat disease. Both are in the clinic and saving lives.
With CRISPR systems, researchers can easily adapt these therapies to target new diseases. However, to do so, physicians must deliver CRISPR systems to cells. Some CRISPR systems are better suited to certain types of delivery than others. At the same time, some delivery techniques are better at targeting certain cells. Thus, having access to a diverse CRISPR toolbox is essential. With a diverse CRISPR toolbox researchers can use more delivery techniques, reach more cells, and treat more diseases.
What are cell and in vivo gene therapies?
In cell therapies, physicians modify cells in the lab. Sometimes they remove disease genes from the cells. Sometimes they make the cells good at killing pathogens. Sometimes they boost the cells’ defenses. Later, they deliver the modified cells to patients.
For instance, sickle cell is a painful blood disease. It is caused by a gene that makes patient’s blood cells deform into sickle-like shapes. In new cell therapies, physicians can alter patients’ blood stem cells in the lab so they no longer form sickle cells. Physicians can then deliver the altered cells to patients to (hopefully) cure the disease.
In in vivo therapies, physicians genetically modify cells already in the body. Often, these therapies alter “disease” genes directly. For instance, physicians can alter genes in the eye to treat some forms of eye disease.
Scientists use CRISPR systems in both cell and in vivo gene therapies. CRISPR systems can carry out the necessary DNA alterations. Scientists must therefore deliver these systems to cells. CRISPR systems can be delivered as DNA, RNA, or ready-to-use protein-RNA complexes. Below, we discuss some of the more popular ways researchers deliver CRISPR systems.
Viral delivery methods
Many gene therapies make use of modified viruses to deliver genes to cells. As with CRISPR systems, these viruses come with their own pros and cons. Some can deliver large cargoes but integrate their cargoes into the genome. Others cause adverse immune reactions. Still others only deliver their cargoes to certain types of cells.
For example, many gene therapies make use of viral delivery vehicles known as adeno-associated viruses (AAVs). AAVs are often considered less immunogenic than other viral vectors. Researchers can also engineer them to deliver DNA to specific kinds of cells. Yet, AAVs have a small “cargo carrying capacity.” That is, they can only carry small amounts of DNA. Indeed, a single AAV cannot carry all of the components necessary for genome editing with the popular SpCas9 CRISPR system. Alternative CRISPR systems can, however, fit into this delivery vehicle.
Other viruses can accommodate much larger CRISPR cargoes, but come with their own drawbacks. For example, adenoviruses can carry a ton of DNA but have caused immune reactions in the past. Lentiviruses can also carry largo cargoes and, while less immunogenic than adenoviruses, can cause problems via insertion of their cargoes into the genome. This unwanted insertion can disrupt genes or regulatory regions and can result in dangerous side effects.
Immunogenicity and random integration are not always an issue. This may be the case in gene therapies directed to immune privileged cells or in cell therapies where it’s possible to check for random integration in the lab respectively. In these circumstances, researchers can use the larger adeno– and lenti– viruses. They can then optimize their CRISPR systems for characteristics other than size. They might, for instance, choose a CRISPR system that can cut their target gene in many places.
Non-viral delivery techniques
Other delivery methods make it possible for researchers to avoid using viruses altogether. These methods often deliver CRISPR systems in the form of ready-to-use proteins/RNAs that edit DNA more efficiently. For example, if researchers are modifying cells in the lab for cell therapies, they can use methods involving chemicals, nanoparticles, or electric pulses to get proteins, DNA, and RNA into cells. With these techniques, scientists can optimize delivery for high efficiency editing and worry much less about immunogenicity and size. They might also choose a CRISPR system that’s particularly easy to purify.
Exploring new CRISPR systems for more delivery options
As you can see, different delivery methods can make use of different CRISPR systems. These systems will always have tradeoffs in terms of things like genome editing accuracy and efficiency. Yet, with access to a diverse CRISPR toolbox, it’s possible to find the right CRISPR systems for the right applications.
At Mammoth we’re excited to find and explore the properties of diverse CRISPR systems. We hope these diverse systems will have huge impacts on CRISPR therapies and beyond.
