Tyler Ford
05/31/2022
Antibiotics have been an essential tool of modern medicine since the 1940s. They’ve cured countless bacterial infections and saved many lives. Unfortunately, many bacteria are evolving resistance to antibiotics. In addition, other microbes like fungi, viruses, and parasites are also evolving resistance to the drugs used to treat them. Researchers call this cumulative problem “antimicrobial resistance.” This growing threat results in more than 1,000,000 deaths per year globally. That number is expected to increase as the impacts of this “silent pandemic” become ever more prevalent.
This series aims to provide a finer point on the impacts of antimicrobial resistance. It highlights some of the antimicrobial resistant pathogens that the US Centers for Disease Control (CDC) considers “Urgent Threats.” These include:
- Neisseria gonorrhoeae
- Candida auris
- Clostridioides difficile
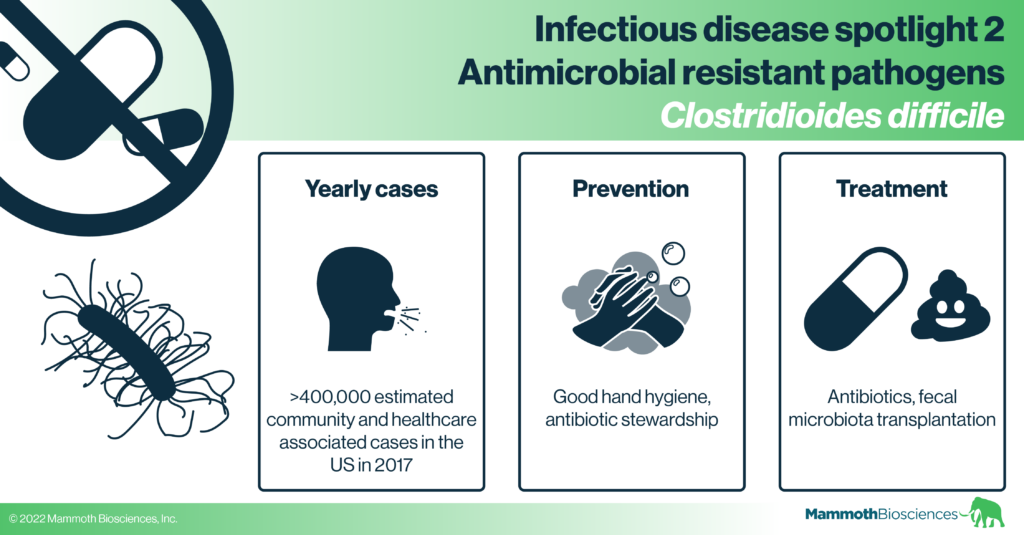
In this post we cover the bacterium, Clostridioides difficile (C. diff). Unlike some of the other microbes associated with antimicrobial resistance, C. diff has not necessarily evolved increased resistance to common antibiotics. Instead, infection with C. diff is a significant consequence of the overuse of antimicrobials known as poor “antimicrobial stewardship.” Many peoples’ guts are asymptomatically colonized by C. diff but these bacteria only occasionally cause dangerous gut infections. Such infections are associated with antibiotic resistance because they often occur after patients have undergone heavy antibiotic treatments for other infections. In these cases, the antibiotics used to treat the unrelated infection destroy the patient’s normal gut bacteria (known as the gut “microbiome”). While these normal bacteria usually keep asymptomatic C. diff in check, their destruction gives C. diff the opportunity to take over the gut. Thus, with poor antimicrobial stewardship, C. diff is given more opportunities to infect the gut.
While physicians can usually treat C. diff infection with antibiotics, in severe cases, C. diff can become antibiotic resistant and recurrent infections arise in many patients. As you’ll learn below, new antibiotics and other forms of treatment like fecal microbiota transplantation may be better at preventing these life-disrupting recurrent infections.
Check out our previous “Infectious disease spotlight” series for posts on the flu, HIV/AIDS, and tuberculosis.
Clostridioides difficile is a common colonizer that occasionally causes severe infections
Clostridioides difficile is a bacterium that often asymptomatically colonizes individuals, and can sometimes cause symptomatic infections. Upon infection, C. diff creates protein toxins that disrupt cells in the gut, leading to diarrhea and inflammation. In extreme cases, C. diff infection can cause the colon to rupture and ultimately results in severe problems like sepsis and even death.
C. diff can be acquired either in the general community or in a healthcare setting, and it is estimated that C. diff caused more than 400,000 infections in 2017 in the U.S. (Guh et al 2020). Outside the body, C. diff can persist in the environment as highly durable spores. Therefore, people can become colonized or infected with C. diff acquired from a variety of sources including contaminated surfaces, contaminated skin, contact with colonized or infected people, contact with colonized or infected animals, and more (Crobach et al 2018). The relative contribution of each of these sources isn’t well understood, but risk factors for symptomatic infection include recent antibiotic use, a recent stay in a hospital or nursing home, age greater than 65, previous C. diff infection, and a weakened immune system (see the CDC website for more info).
Diagnosing C. diff
There are a variety of methods available to diagnose a C. diff infection. As colonization with C. diff is more common than infection, and colonized patients do not necessarily have a C. diff infection, identifying a C. diff infection can be complicated. Often, diagnosis starts with clinical symptoms and a visual analysis of stool samples produced by patients. These efforts are complemented with biochemical and molecular assays (nucleic acid tests). While the analysis of a diarrheal stool sample can identify a patient with a symptomatic bacterial infection, follow-up with biochemical assays that detect C. diff-specific toxin proteins, or molecular tests that detect C. diff’s toxin genes, can confirm the presence of the C. diff bacteria. However, without the visual stool sample analysis, the biochemical or molecular tests alone show only that a patient has C. diff bacteria, but can’t distinguish infection from asymptomatic colonization.
To verify that a patient’s gastrointestinal symptoms are caused by C. diff, the so-called “gold standard” is toxigenic culture. This involves culturing stools samples on nutrients that selectively allow the growth of C. diff and not other microbes. Once colonies grow, their ability to produce toxin proteins is confirmed by a biochemical test or a molecular test. This method is the “gold standard” because it involves direct isolation of C. diff from stool and a confirmation of its ability to produce toxins. However, this method is time consuming. It takes several days as opposed to the mere hours required by biochemical and molecular tests combined with stool analysis (Guery et al 2019). Thus, many prefer the latter method to more rapidly guide treatment.
C. diff treatment
C. diff infection can be treated with a course of antibiotics (usually vancomycin or metronidazole) but resistance can arise and many patients suffer recurrent infections that are both painful and life-disrupting. During treatment for a C. diff infection, antibiotics may wipe out the normal bacteria that reside in the gut. These bacteria constitute the gut microbiome and disruption of the microbiome leaves room for C. diff to continue to grow while also disrupting overall gut health. Indeed, antibiotic treatments for C. diff can clear the infection within 2 weeks, but infection recurs in about 25% of patients. These patients may be treated with additional courses of different antibiotics but these can lose effectiveness over each recurrence (see Mayo Clinic website for more information).
Newer antibiotics may better clear these infections, but, in serious cases of recurrent infection, fecal microbiota transplantation has been demonstrated to be particularly effective (Guery et al 2019). Fecal microbiota transplantation involves the transfer of fecal matter from a healthy individual to the gut of a C. diff infected patient and can be accomplished in a variety of ways including enemas and oral pills (Guery et al 2019). Fecal microbiota transplantation likely works because fecal bacteria from healthy donors colonize the patient gut, restore the microbiome, and prevent C. diff from taking residence. Nonetheless, the precise mechanism and microbes required for this process are unclear. Efforts are underway to develop more defined mixtures of microbes for effective, reproducible treatment of C. diff patients (Guery et al 2019).
Although antibiotic treatment or fecal microbiota transplantation is usually effective, there is still much room for researchers to improve upon or develop new therapies. This is especially true given the high monetary burden of C. diff infection which is estimated to be in the billions annually in the US alone (Gupta and Anathakrishnan 2021). In particular, researchers are working on antibody and bile acid-based therapies for C. diff. Antibody therapeutics bind to C. diff toxins and prevent symptomatic infection. Bile acids normally aid in the digestion of a variety of foods, but it is believed that administering certain types of bile acids can prevent C. diff from taking root in the gut (Guery et al 2019).
Preventing C. diff infection
As C. diff is often passed between patients during prolonged hospital stays, the CDC recommends good hand hygiene (for both patients and care-takers) as a first line of defense against infection. Beyond this, researchers are looking into ways to supplement the healthy microbiome with probiotics that prevent C. diff from growing in the gut. Vaccines based on C. diff toxins are also in clinical development but none have been approved yet.
As C. diff infections often arise in patients who have recently taken antibiotics, scientists are also researching and promoting ways to prevent antibiotics from destroying the healthy gut microbiome. For instance, they are looking into ways to keep antibiotics from killing healthy gut bacteria while still killing their target pathogens. This should block pathogenic C. diff growth (Guery et al 2019). The CDC also promotes antibiotic stewardship as a means of preventing C. diff infections. They hope that stemming the overuse of antibiotics (e.g. mistakenly using antibiotics for viral infections) will lead to less disruption of the healthy gut microbiome and give C. diff fewer opportunities to take over.
The future in the fight against C. diff and other infectious diseases
C. diff infections disrupt lives and kill many people each year. The dangers of C. diff infections are well-known in hospital and long-term care settings. Antibiotics have been a useful, but blunt tool against these and other bacterial infections. As scientists’ understanding of the human microbiome grows, they should be able to develop treatments that specifically prevent “bad” bacteria like C. diff. from flourishing while sparing our normal, healthy bacteria. Coupled with a better understanding of the make-up of a healthy microbiome, researchers will hopefully be able to use their knowledge of our microbial partners to make more specific treatments, reduce suffering, and help many live longer, healthier lives.