Tyler Ford
07/20/2021
When one thinks about therapeutics, often they think of small molecules or proteins. Yet, for many years, scientists have been researching novel ways to use whole cells as drugs. They hope to combat disease with healthy or genetically modified cells that can replace unhealthy cells or attack a disease at its cause.
These “cell therapies” are exciting because they have the potential to cure diseases. In contrast, classical drugs often treat disease symptoms. As a result, patients must take such drugs for their whole lives. Therapeutic cells can (in the best case) grow, divide, and function continuously.
Interestingly, cell therapies aren’t new. One great, older example is a bone marrow transplant. Bone marrow contains “hematopoietic stem cells.” These are the stem cells that produce all the cells in the blood. This includes red blood cells, immune cells, and more. If an individual has dysfunctional hematopoietic stem cells, clinicians can kill off these cells and replace them with healthy bone marrow cells from a matching donor. Such bone marrow transplants have been used to manage or cure primary immunodeficiencies, sickle cell disease, and some forms of cancer.
A prerequisite for this older kind of cell therapy is a matching donor. If the donor and recipient do not match, the recipient may reject the cells. Sometimes the donated cells can also attack the recipient! This can lead to devastating side effects and may even kill the recipient.
Autologous and allogeneic cell therapies
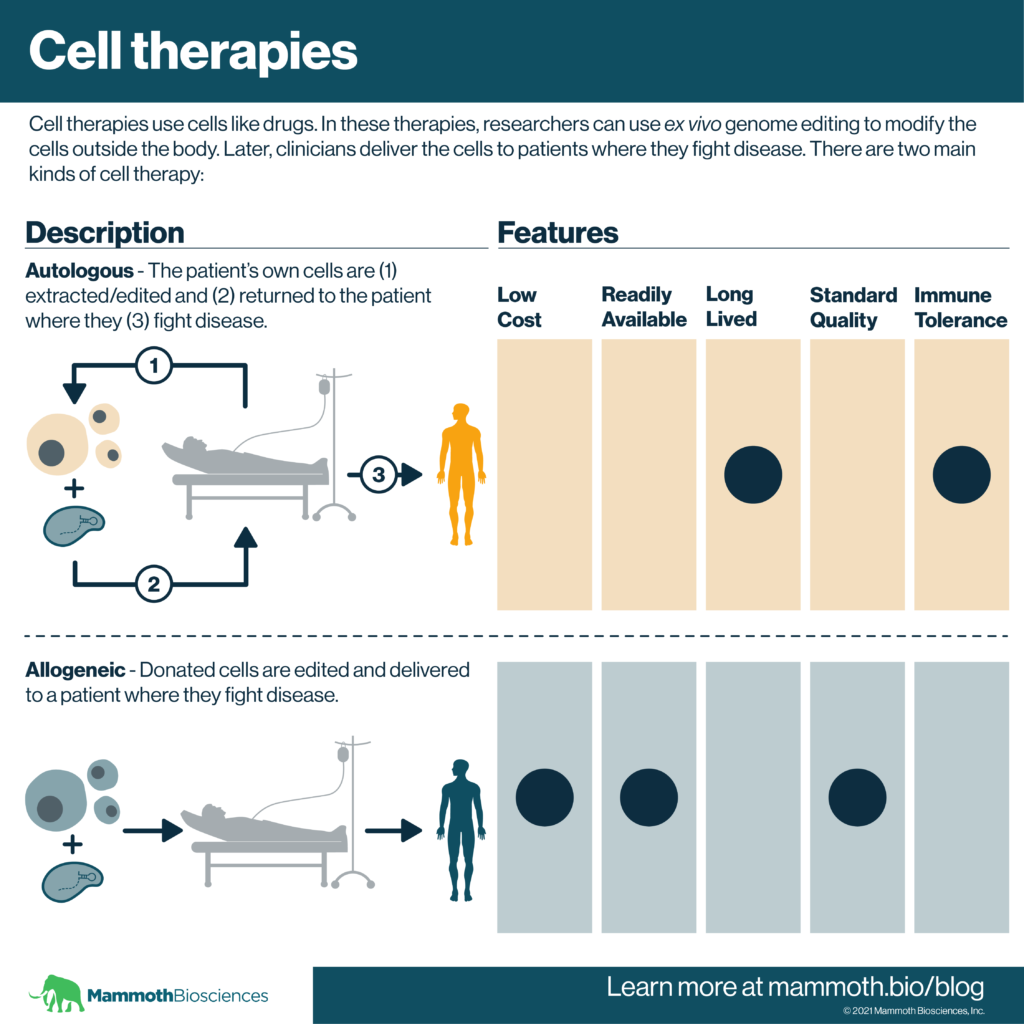
There are two main, newer forms of cell therapy. They involve the isolation of cells either from the patient (autologous) or from a healthy donor (allogeneic). In both cases, researchers can use genetic modification techniques to alter the cells outside the body before they are delivered to the patient. This is considered “ex vivo” genetic modification. In more detail, the two main forms of cell therapy are:
Autologous cell therapies
In autologous cell therapies, the patient’s own cells are turned into therapeutics. Scientists use a variety of techniques to repair or alter the cells’ functions. Because these cells come from the patient, it is unlikely they’ll get rejected.
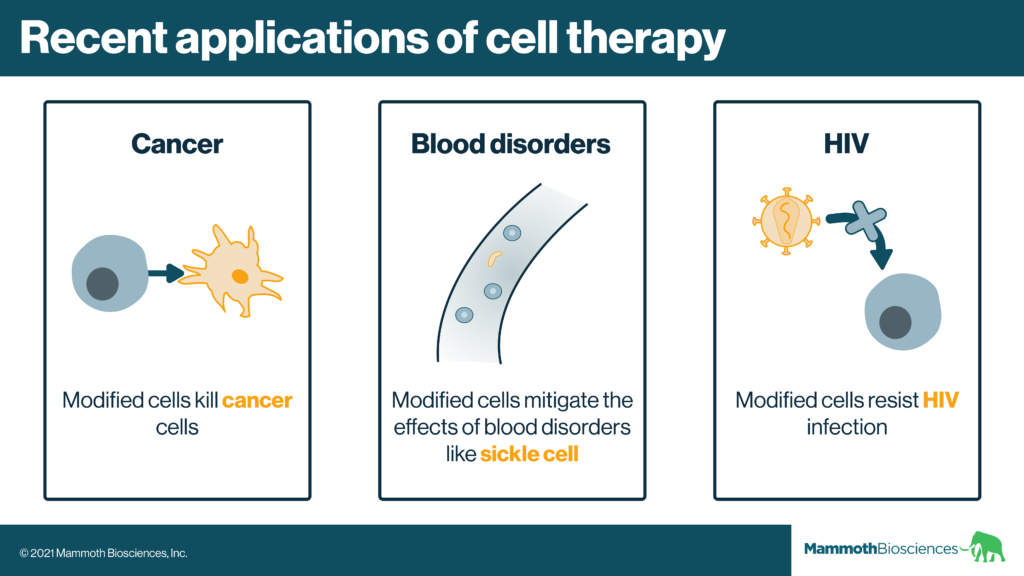
Example: CAR-T cells
CAR-T cells consist of a particular type of genetically modified immune cell. These modified cells can quickly recognize and kill diseased cells (usually cancer cells). Clinicians have used CAR-T cells to effectively treat some aggressive blood cancers.
Autologous cell therapies have been very successful. Yet, they come with a few issues:
- They’re not available on demand. The cells must be isolated and modified for every individual patient. This is time consuming and some patients die as their cells are modified. The supply of autologous cells is also limited if a patient relapses.
- They have variable quality. The cells come from patients who already have a disease. Thus, the final therapeutic product may be contaminated with diseased or non-functional cells. The proportion of functional cells depends on the patient. In fact, in at least one case, contamination of an autologous CAR-T cell product with a patient’s own cancer cells caused cancer relapse (Ruella et al 2019).
- They are expensive. Autologous cell therapies require individualized manufacturing processes. Thus, they can be very expensive. Approved CAR-T cell therapies cost hundreds of thousands of dollars.
Allogeneic cell therapies
“Allogeneic” cell therapies get around some of the issues associated with autologous therapies. Rather than coming from patients, allogeneic cells come from healthy donors. Scientists can use ex vivo genetic modification to make these cells fight disease AND make rejection less likely.
Thus, allogeneic cell therapies should come at a more standard quality. They should also be easier to access on demand or “off the shelf.” Clinical trials using genetically engineered allogeneic cells are underway.
Example: Allogeneic CAR-T cells
As reviewed here, many organizations are conducting clinical trials with allogeneic CAR-T cells. Most of these trials use the allogeneic CAR-T cells to fight blood cancers. In addition, many of them use genome editing to modify the donated cells and make them less likely to attack the patient’s healthy cells.
Ex vivo genome editing and cell therapies
Initial genetic modification technologies used retroviruses and lentiviruses. These technologies insert bits of researcher-designed DNA into cells. However, both insert their cargoes at somewhat random locations in the genome. This limits the precision with which researchers can modify the cells. For retroviruses in particular, this can also lead to dangerous side effects. Indeed, in the past retroviruses have integrated at genome sites that caused modified cells to become cancerous (Tucci et al 2020).
Ex vivo genome editing technologies like CRISPR are a favorable alternative. These technologies precisely integrate engineered DNA sequences at specific genomic locations. They can also precisely delete genes that make cell therapies less effective. Indeed, researchers are already using genome editing to make CAR-T cells more effective (Reviewed in Huang et al 2020).
CRISPR additionally enables researchers to fix or enhance cell functions in more discrete ways. For example, they might make small changes to the cells’ natural DNA. Effective cell therapies for beta-thalassemia and sickle cell use such techniques (Frangoul et al 2021).
As genome editing technologies continue to advance, we’re likely to see more effective cell therapies emerge in the years to come. These technologies may also make it easier to develop effective allogeneic cell therapies.
On the horizon: In vivo genome editing
Ex vivo genome editing results in highly effective cell therapies. Nonetheless, scientists cannot correct all genetic diseases with ex vivo genome editing. It’s just not possible to repair some types of dysfunctional cells and organs by adding new/repaired cells to the body. In addition, cell therapies often require clinicians to kill off patients’ immune cells. This makes it possible for the therapeutic cells to thrive. This can be quite detrimental to the patients and is not viable in all cases. Indeed, such harsh pre-conditioning may prevent second dosing for some cell therapies.
Thus, researchers are actively exploring therapies that use in vivo genome editing. These therapies involve editing large numbers of cells at their natural location in the body. They rely on advances that make it easier to deliver technologies like CRISPR to specific body parts. Such “in vivo” genome editing might one day enable clinicians to treat and cure many more diseases.