Tyler Ford
07/07/2020
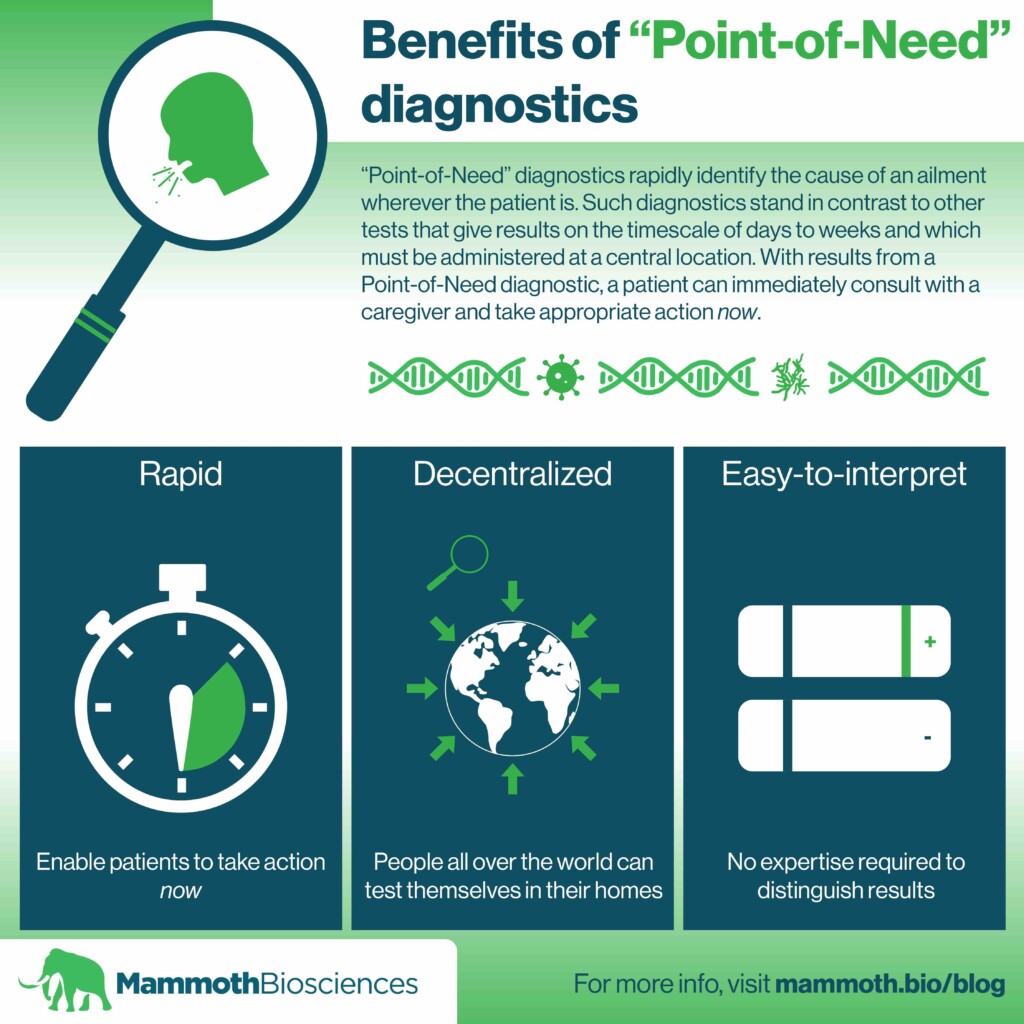
“Point-of-Need” diagnostics rapidly identify the cause of an ailment wherever the patient is. Such diagnostics stand in contrast to other tests that give results on the timescale of days to weeks and which must be administered at a central location. With results from a Point-of-Need diagnostic, a patient can immediately consult with a caregiver and take appropriate action now.
Patients and healthcare workers can particularly benefit from Point-of-Need diagnostics when facing the challenges of infectious diseases like COVID-19. Because of it’s highly infectious nature, it is critical to rapidly diagnose COVID-19 to facilitate the necessary actions to prevent its spread. Healthcare workers can also act rapidly to find anyone who has been in contact with a COVID-19 patient. Thus they can further prevent spread.
At Mammoth, we’re in a development partnership with GSK to develop a Point-of-Need diagnostic device for COVID-19. Importantly however, our CRISPR-based DETECTRTM platform is highly adaptable. We hope to create similar Point-of-Need devices for many infectious diseases.
Current COVID-19 “Point-of-Care” diagnostics
In this post, we distinguish “Point-of-Need” from “Point-of-Care” diagnostics. Point-of-Need diagnostics can be used wherever the patient is. Point-of-Care diagnostics are used in a healthcare facility. Both are rapid, but Point-of-Need diagnostics can be much more widely distributed.
Point-of-Care molecular tests for COVID-19 have already hit the market. However, most of these are insufficient to meet the needs of the pandemic. First, although these diagnostics provide results rapidly, they still require patients to go to a centralized location. Individuals cannot administer the tests themselves. To open the economy and give workers confidence that they’ll be safe, many employers will need their employees to take diagnostic tests either at home or in the office. In addition, it must be easy to distribute diagnostics to places such as nursing homes whose residents can’t easily travel to testing sites.
The wide scale testing afforded by a distributable, portable Point-of-Need diagnostic is particularly necessary for stopping COVID-19. COVID-19 can spread before a patient shows symptoms (the pre-symptomatic stage) and can even spread from patients who show no symptoms (so-called asymptomatic spreaders). Thus, it won’t be enough for symptomatic patients to go to a clinic to get tested. We must make it easy for many people to be tested on a regular basis. Sensitive, specific, portable, and disposable Point-of-Need diagnostics will make this much easier.
Ideal properties of Point-of-Need diagnostics
We’re working to make better Point-of-Need-diagnostics for COVID-19 using our CRISPR-based DETECTRTM platform. With our partners at GSK, we’re aiming to make a diagnostic that is rapid, sensitive/specific, portable, disposable, and easy-to-interpret.
Rapid
In our recent Nature Biotechnology paper, we show that an early version of our CRISPR-based SARS-CoV-2 DETECTRTM can detect SARS-CoV-2 in real patient samples in under 40 minutes. We are currently working to cut this detection time down even further.
Sensitive/specific
In our Nature Biotechnology paper, we also show that the early version of our CRISPR-based SARS-CoV-2 DETECTRTM has 95% positive predictive agreement and 100% negative predictive agreement when compared to the CDC’s PCR-based test for the disease. Additionally, our test showed no cross-reactivity with other closely related viruses. This is a great starting point and we’re working to improve the final product even further.
Portability
We are working to make our diagnostic device small, inexpensive, and easy to transport. It will be easy to use and self-contained. This will enable distribution to homes, offices, and healthcare facilities. Users will simply have to apply a sample directly to the device and wait 30 minutes or less for a result.
Disposable
We are working to develop this and other diagnostics using inexpensive, disposable materials. Thus, we will make it possible to distribute our diagnostics to the underserved communities and locations that have been hardest hit by the pandemic.
Easy-to-interpret
Much like an at-home pregnancy test, a Point-of-Need diagnostic must be easy-to-interpret. Patients must be able to understand the results with no expertise and thus be able to take the appropriate action. We plan to make the results of our COVID-19 diagnostics and all future diagnostics easy for anyone to understand.
Giving people peace of mind from their own home
With a Point-of-Need diagnostic we can more effectively control and halt the COVID-19 pandemic. We hope our ultimate product will be of great use to healthcare workers, patients, and epidemiologists around the world. We also hope to make it accessible to anyone who wants it. Thus, people will get peace of mind from COVID-19 testing in the comfort of their own homes.
Learn about our CRISPR-based SARS-CoV-2 detection platform for commercial labs!
