Tyler Ford
06/17/2020
In order to slow and stop the COVID-19 pandemic, we need to be able to quickly and accurately determine who is currently infected and who has been infected with SARS-CoV-2. With these key pieces of information, we can identify hot spots for infection. We can also prioritize the allocation of resources to those who are most at risk. We can even loosen or tighten social restrictions in more effective ways.
There are 3 main types of COVID-19 tests available. These are:
- Nucleic acid (molecular) tests
- Antigen tests
- Serological (antibody) tests
Each type of test has its own special features and uses. Indeed, as SARS-CoV-2 infection progresses, we need different diagnostic solutions. While PCR and antigen tests are useful for detecting SARS-CoV-2 during the initial stages of infection, serological tests are useful during the late stages and post infection.
At Mammoth, we’re making CRISPR-based nucleic acid tests that are available at the point-of-need. We discuss all three kinds of tests in detail below.
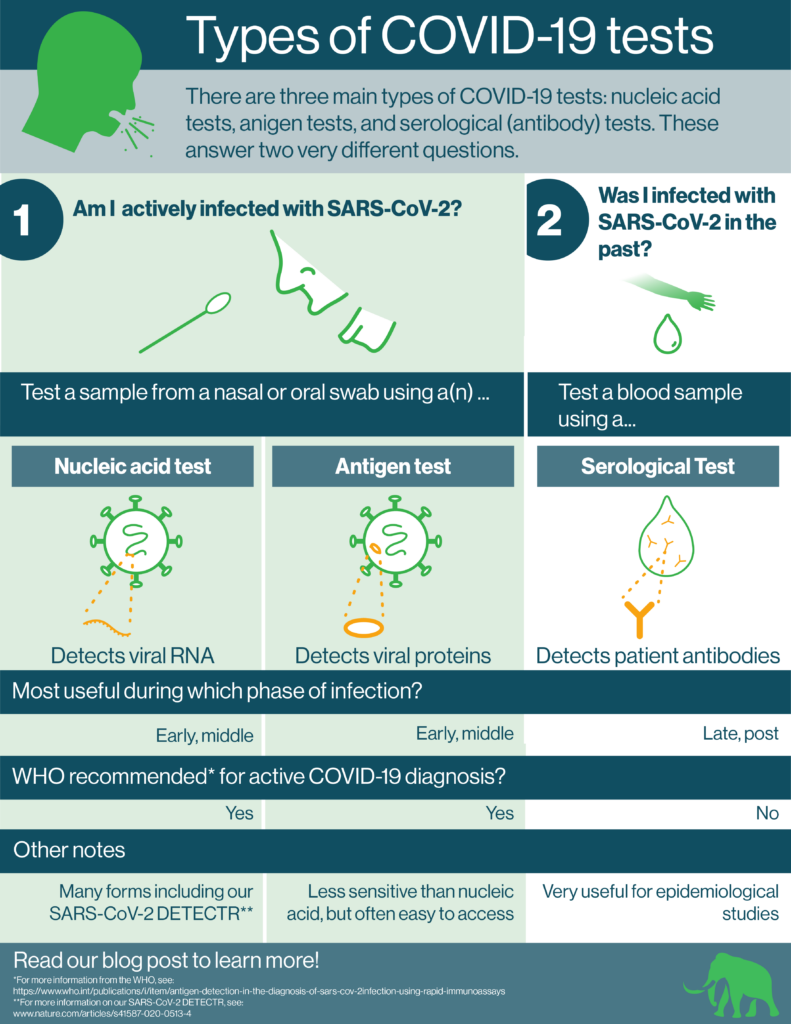
The 3 main types of COVID-19 tests: nucleic acid, antigen, and serological
We can break down the 3 types of COVID-19 tests according to their use. Then, we can divide them further according to the compounds they actually detect. Nucleic acid and antigen tests are used to diagnose active infection. They look for components of the SARS-CoV-2 virus itself. They look for these parts in nasal or oral swabs. Nucleic acid tests look for the SARS-CoV-2 RNA genome. Antigen tests look for proteins that make up the virus.
Serological tests (also called antibody tests) determine if a person was infected in the past. For these tests, healthcare workers take blood samples from patients. Then, they check whether these samples contain antibodies that bind to SARS-CoV-2. The presence of such antibodies indicates that the patient mounted an immune response to SARS-CoV-2 because they were infected in the past. This does not necessarily mean that the patient is immune to future SARS-CoV-2 infection.
Pros and Cons of COVID-19 tests
Like all assays, each type of COVID-19 test has its own pros and cons. All three are necessary for controlling the pandemic.
Nucleic acid tests
Nucleic acid tests usually make use of a targeted nucleic acid amplification technique such as the polymerase chain reaction. Such amplification techniques turn the small amount of viral genomic material in patient samples into large amounts of DNA. Researchers can use a variety of techniques to detect these large amounts of DNA.
Because of the targeted amplification step, nucleic tests are usually highly specific and very sensitive. They can be used to detect infection early and remain capable of detecting the SARS-CoV-2 genome weeks into infection. Yet, they can also be time consuming. Sometimes they also require bulky lab equipment.
Our CRISPR-based SARS-CoV-2 DETECTR is a nucleic acid test that overcomes the negatives of standard nucleic acid tests. It combines nucleic acid amplification with CRISPR-based detection. CRISPR-based detection both enhances specificity and amplifies signal further. As a result, less time is required for the whole test. In its current form, the test requires no bulky lab equipment. We aim to make it portable, disposable, and capable of producing results at the point-of-need. Thus, patients and healthcare workers will be able to use the test at decentralized locations and act on the test’s results quickly. Learn more here.
Antigen tests
Antigen tests use antibodies to detect protein components of the SARS-CoV-2 virus. These tests are often rapid and easy to administer. However, they’re usually less sensitive and less specific than nucleic acid tests. The WHO recommends that those who are at high risk of infection or who are symptomatic use antigen tests to quickly diagnose an infection. Results can later be confirmed with nucleic acid tests if available. Confirmation of positive results with a nucleic acid test is particularly recommended when case numbers are low.
Serological (antibody) tests
Serological tests detect whether or not a person has developed antibodies against various components of the SARS-CoV-2 virus. Because these antibodies are developed during the course of infection or as a result of vaccination, serological tests are not generally positive during the early stages of infection and have low sensitivity during these early stages. Thus, serological tests should not be used to detect active infection.
Nonetheless, serological tests can be incredibly useful to epidemiologists. They can rapidly use these tests on large populations. With the resultant number of positive tests and the known specificity/sensitivity of the tests, they can estimate how many people in a given population have been infected. Such information may be useful when officials decide whether or not to lift restrictions.
Test test test
All three kinds of COVID-19 tests will be useful to slow and stop the COVID-19 pandemic. Combined use of nucleic acid and antigen tests can make us more confident in our diagnoses. They enable patients and healthcare workers to treat and isolate patients who are infected and who may develop serious disease now. Serological tests can help epidemiologists understand how many people have been infected and may make it easier to assess the effectiveness of COVID-19 treatments and vaccines.
Mammoth and other organizations will continue improving these tests to make their administration and deployment easier. In particular, we believe it is critical to make point-of-need diagnostics that identify infection rapidly. With results from a point-of-need diagnostic, a patient can consult with a care-giver to determine what actions they can take in real time. Widely available point-of-need diagnostics will make it possible to control not only COVID-19 but many other devastating infectious diseases.
Learn about our CRISPR-based SARS-CoV-2 detection platform for commercial labs!
